About KEYTRUDA
About KEYTRUDA®
(pembrolizumab)
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland) [External links]
Please refer to the KEYTRUDA Summary of Product Characteristics and Risk Minimisation Materials before prescribing KEYTRUDA. Risk Minimisation Materials are available online, from your MSD representative or from MSD Medical Information (Email: medicalinformationuk@msd.com, Phone: 0208 154 8000).
KEYTRUDA licensed indications

KEYTRUDA, in combination with chemotherapy with or without bevacizumab, is indicated for the treatment of persistent, recurrent, or metastatic cervical cancer in adults whose tumours express PD-L1 with a CPS ≥1.

KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma who have failed autologous stem cell transplant (ASCT), or following at least two prior therapies when ASCT is not a treatment option.

KEYTRUDA as monotherapy is indicated for adults with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer in the following settings:
- first-line treatment of metastatic colorectal cancer;
- treatment of unresectable or metastatic colorectal cancer after previous fluoropyrimidine-based combination therapy.

KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of advanced or recurrent endometrial carcinoma in adults who have disease progression on or following prior treatment with a platinum-containing therapy in any setting, and who are not candidates for curative surgery or radiation.
KEYTRUDA, as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma in adults whose tumours express PD-L1 with a CPS ≥1.
KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic head and neck squamous cell carcinoma in adults whose tumours express PD-L1 with a ≥50% TPS and progressing on or after platinum-containing chemotherapy.
KEYTRUDA as monotherapy is indicated for the treatment of adults and adolescents aged 12 years and older with advanced (unresectable or metastatic) melanoma.
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults and adolescents aged 12 years and older with Stage IIB, IIC or III melanoma and who have undergone complete resection.

KEYTRUDA as monotherapy is indicated for the treatment of the following microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) tumours in adults with:
- advanced or recurrent endometrial carcinoma, who have disease progression on or following prior treatment with a platinum-containing therapy in any setting and who are not candidates for curative surgery or radiation;
- unresectable or metastatic gastric, small intestine, or biliary cancer, who have disease progression on or following at least one prior therapy.
First-line indications
KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous non-small cell lung carcinoma (NSCLC) in adults.
KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous non-small cell lung carcinoma (NSCLC) in adults whose tumours have no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) positive mutations.
KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) positive tumour mutations.
Previously treated indication

KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA.
KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line treatment of patients with locally advanced unresectable or metastatic carcinoma of the oesophagus or HER-2 negative gastroesophageal junction adenocarcinoma in adults whose tumours express PD‑L1 with a CPS ≥10 (see section 5.1).
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.
KEYTRUDA is indicated in combination with axitinib for the first-line treatment of advanced renal cell carcinoma (aRCC) in adults.
KEYTRUDA, in combination with lenvatinib, is indicated for the first-line treatment of advanced renal cell carcinoma (aRCC) in adults.
KEYTRUDA, in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advanced, or early-stage triple-negative breast cancer at high risk of recurrence.
KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple-negative breast cancer in adults whose tumours express PD-L1 with a CPS ≥10 and who have not received prior chemotherapy for metastatic disease.
KEYTRUDA is a selective monoclonal antibody that blocks the programmed cell death-1 (PD-1) protein pathway, potentiating T-cell responses, including anti-tumour responses.1,2,3
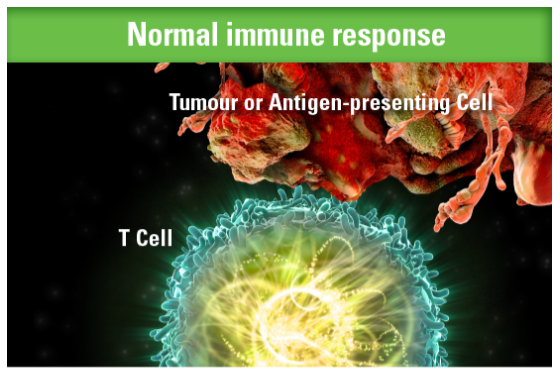
When functioning properly, T cells are activated and can attack tumour cells1,2,3
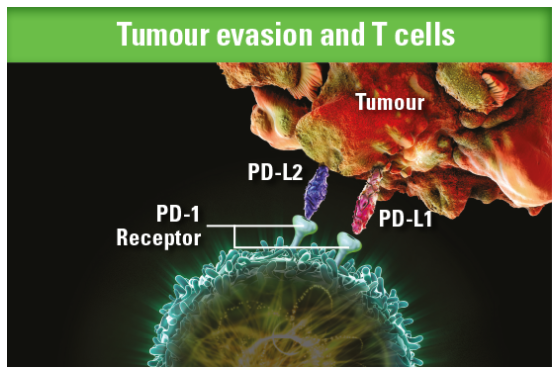
Some tumours can evade the immune system through the PD-1 pathway. On the surface of tumour cells the dual PD-1 ligands, PD-L1 and PD-L2, bind to the PD-1 receptors on T cells to inactivate them, allowing tumour cells to evade detection1,2,3

By inhibiting this process, KEYTRUDA reactivates tumour-specific cytotoxic T lymphocytes and anti-tumour immunity1,2,3
PD-1 = Programmed Cell Death-1; PD-L1 = Programmed Cell Death Ligand-1; PD-L2 = Programmed Cell Death Ligand-2.
References
- Harvey, RD. Clin Pharm Therapeutics. 2014:96(2):214-223.
- KEYTRUDA Summary of Product Characteristics (Great Britain).
- KEYTRUDA Summary of Product Characteristics (Northern Ireland).
Supporting documentation
Prescribing Information (Great Britain) & Prescribing Information (Northern Ireland)
By clicking the links above you will leave the MSD Connect website and be taken to the emc PI portal website
GB-PDO-02299 | Date of Preparation: August 2022

